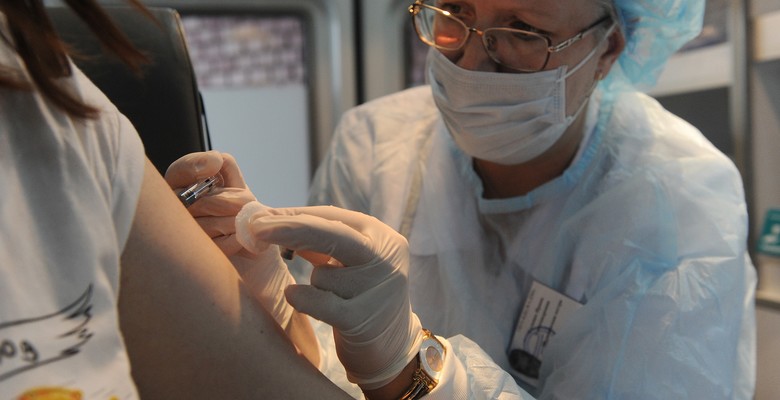
Every year flu epidemics spread around the globe. They are so common that with influenza the word “pandemic” meaning a global epidemic has lost its original meaning. Nowadays, pandemic refers only to such epidemics that are caused by especially dangerous virus modifications (the Spanish flu, Hong Kong flu, avian flu and swine flu). This year has been no exception. We are at the brink of an ordinary routine pandemic, which so far does not show any signs of abnormal level of mortality.
Influenza virus is so labile that there is no way to fully exterminate it, as it is constantly changing faster than the newest flu medication and vaccines. Drugs and vaccines are constantly improved as well, and sooner or later universal medication and vaccines will make it possible to solve the flu problem and terminate the large-scale epidemics. Applying modern nanotechnologies in vaccine production is a promising breakthrough in this race.
Flu medication
A patient who already caught the flu is treated with antiviral drugs. Doctors call it causal treatment, i.e. treatment aimed at removing the cause of the disease. Causal therapy includes substances that are capable of damaging (inactivating) the specific elements of influenza virus usually located at its surface, in the viral envelope. Such drugs are especially effective at early stages of infection when the virus only begins to multiply in the patient’s body.
Despite a large array of chemical medications that suppress flu virus reproduction, such drugs are not always effective in combatting the flu virus. That is logical as application of causal therapy aimed at a specific link in the process of virus replication entails the selection of the virus strains resistant to such treatment. Consequently, after applying highly specific drugs for one flu season the detection rate of resistant strains reaches 5-40%.
A more promising method includes the prevention of influenza and its severe consequences via vaccination. Injection of a vaccine causes an immune response in the body: the body starts producing antibodies to the surface antigens (hemagglutinin and neuraminidase) as well as to other viral structure components. Vaccination provokes our bodies to start producing our internal drugs against the flu virus.
Vaccination Pros and Cons
Today there are quite a number of flu vaccines, which can be divided into four groups based on their production method.
The positive sides of vaccination (ability to form immune response to the virus) are described by the notion of immunogenicity. The negatives (sides effects after immunization) are described as reactogenicity. The relationship between these characteristics determines the quality of a vaccine, its efficacy and safety.
Unfortunately, these characteristics – immunogenicity and reactogenicity – are interlinked: the higher the efficacy of a vaccine, the higher is the risk of side effects on the body. It is typical of all vaccines regardless of their type – be it a live inactivated, split or subunit vaccine; polyvalent (usually a trivalent vaccine against the two seasonal strains of type A and one type B flu) or pandemic (against the specific strain with increased virulence, e.g. swine flu).
Clearly, researchers aim at developing vaccines with maximum immunogenicity and minimum reactogenicity. World’s leading laboratories study various types of viral material and methods of processing, including utilization of auxiliary supplements.
Supplement syndrome
As an auxiliary supplement a vaccine can include preservatives, buffer components and enhancers of immune response (the so-called adjuvants). Examples of adjuvants include oil emulsions, lipopolysaccharide globules, inorganic nanoparticles, polymers or their combinations. Foe example, recently Sanofi Pasteur (producer of the Vaxigrip vaccine) patented a product containing nanoparticles of aluminum hydroxide stabilized by polyacrylate. Derivatives of fullerene and precious metals nanoparticles are also known adjuvant examples.
Adjuvants enhance the immunogenicity, but themselves can cause side effects. In other words, existing adjuvants increase the reactogenicity of vaccines. Recently, the term ASIA (Autoimmune/Inflammatory Syndrome Induced by Adjuvants) was coined. The most frequent manifestations of this syndrome are fever, joint pain, dermatosis, weakness and muscular pain.
Structural modification of vaccines
Our research group has modified a flu vaccine with the help of nanoparticles of ceric oxide. One of the key characteristics of nano-crystal ceric oxide is its ability to inactivate active forms of oxygen and prevent oxidation stress. This feature makes it a safe adjuvant not leading to ASIA.
For basis we took a trivalent split vaccine Vaxigrip (Sanofi Pasteur, France) that consists of inactivated split A/H1N1 and A/H3N2 viruses, as well as type B virus. The vaccine was modified by adding nanoparticles of ceric oxide of the 2-6 nm size. The immunogenicity of the modified vaccine was studied on white mice. Specialists can view the details of our work in the March 2016 issue of the international journal Antiviral Research, while in this article we are going to present only the practical results.
Animals immunized with non-modified vaccine exhibited a four-fold increase in A/H1N1 and A/H3N2 flu virus antibodies, but in respect to the type B virus the level of antibodies did not differ from that in non-immunized animals. Thus, while the immunogenicity of the Vaxigrip vaccine for type A viruses meets the flu vaccine requirements of the European Committee, it is not the case for the type B virus.
The group of mice immunized with the nanoparticle modified vaccine showed a significant increase in antibody titers towards all three types of flu virus included in the vaccine. In respect to the A/H1N1 and A/H3N2 flu viruses the increase in antibody level was eight-fold, while in respect to type B flu virus (notorious for its low levels of immunogenicity) the increase was four-fold!
Vaccine modification with nanoparticles did not entail any increase in reactogenicity, but increased the duration of the circulation of antibodies in the bloodstream of immunized animals, which was supposed to provide a longer protection from influenza infection.
The role of the CeO2 nanoparticles becomes obvious if we study the vaccine they modify in electronic microscope. A regular Vaxigrip split vaccine looks like an unordered set of viral fragments. Injecting ceric oxide nanoparticles into its content leads to the nanoparticle adsorption by the viral membranes and formation of larger particles that contain fragments of various viruses. The experiments reveal that such structure makes the vaccine more effective.
The developed method of vaccine modification is simple and inexpensive and does not require radical technological changes in the vaccine production. In order to obtain a modified vaccine we simply add to its content a certain proportion of ceric oxide nanoparticles. Subsequently, the viral fragments organize themselves into multicomponent structures.
This method requires further in-depth research. But it is already clear that use of ceric oxide based nanostructures in vaccines is a promising method of increasing the specific activity of flu vaccines.
Vladimir Ivanov,
Corresponding member of the Russian Academy of Sciences,
The Kurnakov Institute for General and Inorganic Chemistry of the RAS
Nadezhda Zholobak,
PhD in Biology
Alexander Scherbakov,
PhD in Chemistry








